Structure and function of the sodium/proline symporter PutP
Structure and function of the sodium/proline symporter PutP
The sodium/proline transporter PutP is a member of the sodium/solute symporter (SSS) family (TC 2A.21, SLC5), which contains more than 1000 proteins of pro- and eukaryotic origin. Within the family, the capability of proline uptake is restricted to proteins of bacteria and archaea. Here, proline uptake via PutP may contribute to: i. the use of proline as source of carbon, nitrogen and energy; ii. the supply of cells with compatible solute during adaptation to osmotic stress; or iii. the modulation of the intracellular redox environment and scavenging of reactive oxygen species. Based on these functions, PutP can be of significance for bacteria-host interactions including the virulence of pathogens like as Helicobacter pylori, Staphylococcus aureus and others.
We use PutP as a model to obtain insights into the structure and function of SSS family proteins. To experimentally analyze structural features of PutP, site-directed spin labeling in combination with electron paramagnetic resonance (EPR) spectroscopy is employed. Based on pulse EPR distance measurements, a coarse-grained model of the packing of the 13 TMS of PutP is generated. In addition, the EPR-based distances analysis was used to determine the backbone structure of an individual TM. Guided by a vSGLT-based homology model, a comprehensive analysis of structure-function relationships in PutP is performed. The analysis led to the identification of the putative sites of sodium and proline binding. Furthermore, information on the ligand translocation pathway and functional relevant conformational alterations of the transporter is obtained by cysteine accessibility analyses. Our results support a mechanistic model according to which transport follows an alternating access mechanism.
Cooperations: Prof. Dr. G. Jeschke, Physical Chemistry, ETH Zurich
Prof. Dr. H.J. Steinhoff, Dept. Physics, University Osnabrück
Prof. Dr. H. Michel, MPI of Biophysics, Frankfurt
Dr. E. Olkhova, MPI of Biophysics, Frankfurt
Financial support: DFG
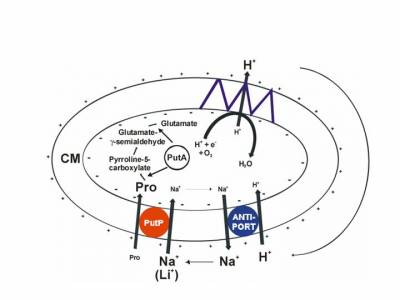
Fig.: Proline transport metabolism in Escherichia coli

